A Brief Intro - Ortho Implants: Scw, Plt & Nails
A BRIEF INTRODUCTION INTO ORTHOPEDIC IMPLANTS: SCREWS, PLATES, AND NAILS
GHOLAMREZA ROUHI1,2, MOHSEN AMANI1
1 Assistant Professor, Faculty of Biomedical Engineering, Amirkabir University of Technology, Tehran, Iran.
2 Adjunct Professor, School of Human Kinetics, Faculty of Health Sciences, University of Ottawa, ON, Canada.
1 Graduate Student of Biomedical Engineering, Amirkabir University of Technology, Tehran, Iran.
Introduction:
Bone, as a hard biological tissue, consists of cells resided in the bone matrix, which is made by an organic (primarily collagen (90%) and 10% amorphous ground substance) and a mineral phase. The main constituents of bone mineral are calcium phosphate and calcium carbonate. The mineral components consist mainly of hydroxyapatite crystals and amorphous calcium phosphate. Bone acts as a reservoir for our body's calcium, also serves as a protector for vital organs, as well as provides mechanical stability to our body, and makes locomotion possible. This hard biological tissue has a hierarchical structure and is designed optimally. There are two types of bone, at the macroscopic level, in our body: cortical (compact or Haversian) and cancellous (spongy or trabecular) bone (see Fig. 1). This specific classification is based on the difference in their microstructure and basic units, as well as in the extent of their porosities. Cortical bone's porosities are at the micro-scales (the size of voids are on the order of a couple of micrometers, and the porosity of cortical bone varies from 5 to 30%), while cancellous bones' porosities range from 30% to more than 90% [1].

Fig. 1. Cortical (compact or Haversian) and cancellous (spongy or trabecular) bone in femur (left) and vertebra [2, 3].
Based on the arrangements of the collagen fibrils, compact and spongy bones can be classified either as woven or parallel-fibered type. Woven bone, also called coarse-fiber bone, is characterized by the presence in the matrix of coarse, irregularly oriented collagen fibrils. Woven bone is the bone formed during skeletal embryogenesis, and after birth it is gradually removed by the process of bone remodeling, and is substituted by lamellar bone. It should be noted that the woven bone be formed in pathological conditions such as callus formation, as well, It can be said that woven and lamellar bone are the result of a rapid and a slow osteogenic process, respectively. On the other hand, parallel-fibered bone consists of relatively thin and parallel-oriented collagen fibrils. Lamellar bone may be regularly organized into unit layers called lamellae.
Bone can gain maximum strength with minimum mass due to continuous activities of various kinds of bone cells, i.e. osteocytes, osteoblasts, osteoclasts, and bone lining cells. There are continuous processes of bone resorption and formation in our bones from birth to death, which is so-called, bone remodeling process [4]. Bone remodeling process aims to provide maximum strength with minimum mass to our bones. When a bone is broken, there is no other way than fixing it by employing man-made supportive structures. Fortunately, bone has a great capability in re-gaining its lost strength through the healing process.
The healing process of bone is a complex process in which both medicine and mechanics are greatly at play and they can alter the time course of the healing process. Interesting to note that all broken bones go through the same healing process. The bone healing process has three stages: inflammation, bone production (soft callus formation stage, and hard callus formation stage), and bone remodeling. The inflammation stage begins the moment the bone is broken and lasts for around five days. Fortunately bone has a very good blood supply due to the channels within its structure. When a fracture occurs, there is massive disruption to these blood channels and a large amount of bleeding appears from the fracture fragments. This is what causes immediate swelling and bruising in the area of the broken bone. This is known as a Hematoma, which means bleeding within the tissue. The damaged bone tissue at the edges of the fracture fragments die back and the dead cells release chemicals called cytokines, which initiate the healing process. Within hours of the fracture, the blood from the fracture fragments forms a mesh of clotted blood, which is the first link between the two fragments and contains special cells called fibroblasts. Fibroblasts begin to lay down tissue called granulation tissue between 4 and 10 days after the fracture occurs. The granulation tissue forms a scaffold between the two fragments from which the formation of a soft callus can begin. Fibroblast cells present in the granulation tissue begin to form cartilage and fibrocartilage. This is a spongy material that fills the gap between the two fracture fragments, although it is quite weak to external mechanical stimuli for around six weeks. For this reason it is important that there is not too much movement of the fracture fragments at this stage. After a couple of weeks, despite the very fragile nature of soft callus, it offers sufficient stability at the fracture site for new blood vessels to begin forming and for osteoblasts at the periosteum (the outer surface of the bone) to begin laying down what is called woven bone. This woven bone at the margins of the fracture is a little soft and disorganized, but it makes the first bone contact between the two fracture fragments.
From two to three weeks onwards a process begins whereby the fragile cartilage material of the soft callus is transformed completely into woven bone. This process typically continues for between six and twelve weeks, depending on the location and type of fracture (generally six weeks for the upper limb and twelve weeks for the lower limb). Hard Callus formation is a complex process that is guided by the release of mineral compounds such as Calcium and Phosphate into the Cartilage tissue, which subsequently transforms into a bridge of Hard Callus over the fracture site. Once the hard callus has formed at the fracture site, then fracture union is said to have occurred. Fracture union can be seen on x-ray at around six weeks in upper limb fractures and twelve weeks in lower limb fractures. During normal bone healing the body will lay down harder callus than is needed, and as a result the fracture site enlarges. Bone remodeling begins once the fracture has united and may continue for several years. Through the remodeling process and over time, the normal shape of the bone is regained. Bone is resorbed where is not needed by osteoclasts and formed by osteoblasts where is needed. At the bone remodeling stage of fracture healing, a progression of weight bearing exercise is encouraged because it leads to an increase in bone strength. During the course of the bone remodeling process, the loosely organized woven bone is gradually replaced by lamellar bone, which is highly organized and much stronger than woven bone.
Orthopedic implants can be defined as medical devices used to replace or provide fixation of bone, or to replace articulating surfaces of a joint. In simpler words, orthopedic implants are used to either assist or replace damaged or troubled bones and joints. Orthopedic implants are mainly made from stainless steel and titanium alloys for strength and lined with plastic to act as artificial cartilage in order to reduce the stress at the articulating surfaces. Some implants are cemented into place and others are pressed to fit, so that your bone can grow into the implant for strength. Some examples of orthopaedic implants are: orthopaedic plates, orthopaedic nails, and orthopaedic screws. The key factor that guides bone healing is the interfragmentary movement, which determines the tissue strain and consequently the cellular reaction in the fracture healing zone. Thus, the methods of fracture fixation will be evaluated by considering their ability to reduce the interfragmentary movement. To achieve good and acceptable healing results, biomechanical principles should be understood and carefully taken into consideration.
Orthopedic Screws:
The helical-thread screw is surely an extremely important invention in Mechanical Engineering, which changes angular motion to linear motion to transmit power, or to develop large forces. Screws are complex tools with a four-part construction: head, shaft, thread, and tip. The head serves as an attachment for the screw driver, which may be hexagonal, cruciate, slotted, or Philips in design. The head also serves as the counterforce against which compression generated by the screw acts on the bone. The shaft or shank is the smooth portion of the screw between the head and the threaded region. The thread is defined by its root (or core) diameter, its thread (or outside) diameter, its pitch (or distance between adjacent threads), and its lead (or distance it advances into the bone with each complete turn) (see Fig. 2). The root area determines the resistance of the screw to pull-out forces and relates to the area of the bone at the thread interface and the root area of the tapped thread. The cross-sectional design is usually a buttress or V-thread (usually used in machine screws) (see Fig. 3). The tip of the screw is either round (requires pretapping) or self-tapping (fluted or trocar).

Fig. 2. Screw terms definition [5].

Fig. 3. Buttress (left) and V-shape (right) screw thread profile [5].
Orthopaedic screws are the most commonly used devices for bone fracture fixation. They are used as both standalone fixators and in conjunction with other orthopaedic hardware devices, particularly plates. Screws are primarily responsible for retaining the stability of most screw-plate fixation devices and are commonly associated with failure due to pull-out associated with poor screw purchase or bone loss. Therefore, particular attention should be placed on the type of screws in use and their placement in bone. Primarily responsible for maintaining the stability of plated-bone constructs, and supplying necessary interfragmentary compression, screw-hold in bone is very crucial. It is well known that geometric parameters influence the pullout strength of orthopaedic screws [6-8], however their effect on the long-term bone-screw interaction is largely unknown. Pullout tests performed in vivo and in synthetic samples [6-9] have suggested a link between a screw’s geometry, material properties, and its pullout strength. As expected, the resistance to bone shearing proves to be predominantly reliant upon the density of the host material [10]; however, the screw’s outer diameter and length of engagement critically affect the holding strength of screws in bone [6, 7]. Other characteristics, such as the screw’s pitch, inner diameter, and thread profile shape contribute to the holding power as well, but to a lesser degree [6-8]. Although sufficient pullout strength is necessary to prevent initial screw avulsion, it is not a good indicator of potential stress shielding effects. Clinically, if pull-out of the screw is a concern because of soft bone, a larger thread diameter may be preferred, whereas if the bone is strong and fatigue is more of a concern, a screw with a wider root diameter will have a higher resistance to fatigue failure [10].

The use of a screw to convert torque forces to compression forced across a fracture is a valuable technique. Its success requires application of the screw in a manner that allows gliding of the proximal portion of the screw in the near bone and threads purchase in the opposite cortex, so that the head of the screw will exert load and force the fracture together. Careful selection of the screw angle respective to the fracture is necessary to prevent sliding of the fracture fragments as they are compressed (Fig. 4) [10].
The basis of bone-plate fixation relies on the compression of the plate to the bone by the induced tensile stresses in the screw [11]. An approximately linear relationship exists between the insertion torque applied to the screw and the resulting tension. The screw should be inserted at the highest possible torque (without shearing the bone), in order to induce a desired higher screw tension. A higher screw tension is desirable because a higher frictional force must be overcome in order for loosening to occur, and it will also likely result in an increased transfer of mechanical stimuli to bone, which will reduce stress shielding.

Figure 4 Interfragmentary compression orthopedic screw [12].
Since the screw remains attached to the bony tissue after it is healed, it may diminish the bone’s strength and stiffness. The significantly stiffer metallic screw (elastic modulus of 100 to 200 GPa) carries most of the shared load, causing the adjacent bone (elastic modulus of 1 to 20 GPa for cancellous and cortical bones, respectively) to be atrophied in response to the diminished load it is carrying, in accordance with Wolff’s law of functional adaptation (see Fig. 5). Wolff’s law states that “Every change in the form and function of a bone is followed by certain definite changes in their internal architecture and equally definite secondary alterations in their external conformation, in accordance with mathematical laws”. The effect of metallic screws on the bony tissue in their vicinity is called ‘stress shielding’. The ‘biomechanical compatibility’ of a particular screw with bone can, therefore, be characterized by the stress (or strain, or any other mechanical stimulus) distribution developing in the bone around the screw as a result of the screw’s tightening during implantation[13]. Screw loosening is a common problem faced in bone fracture fixation. Stress shielding around screw threads is partially responsible for excessive bone resorption. Research related to bone loss around screw threads is an under-studied topic, and a great deal of consideration must go into the design of implants in order to reduce this pitfall. This is particularly true in the case of brittle fractures associated with low density osteoporotic bone [14]. In these cases it is probable that the rate of bone resorption in the vicinity of implants will be higher than that of a similar fracture in a healthy bone, due to its already weakened state.

Fig. 5. An illustration of the process leading to failure of the fixation (screw loosening) as a result of lack of enough mechanical stimuli to the neigboring bone (the stress-shielding effect), and thus excessive bone resorption around the screw threads [13].
Histological evidence has shown that active compression of the bone-screw interface resulted in little activity at the site of compression, whereas neutral regions wherein a gap exists between the screw and bone, exhibited areas of high activity, i.e. resorption of necrotic bone followed by formation of new bone [15].
Orthopedic Plates:
Plate and screw fixation of fractures has undergone continual design modifications and improvements during recent decades. Friedrich Pauwels for the first time defined and applied the tension band principle in the fixation of fractures and nonunions. This engineering principle applies to the conversion of tensile forces to compression forces on the convex side of an eccentrically loaded bone. This is accomplished by placing a tension band (bone plate) across the fracture on the tension (or convex) side of the bone. Tensile forces are counteracted by the tension band at this position and converted into compressive forces. If the plate is applied to the compression (or concave) side of the bone, it is very likely to bend, fatigue, and fail. Therefore, a basic principal of tension band plating is that it must be applied to the tension side of the bone, so that the bone itself will receive the compressive forces (Fig. 6) [10].
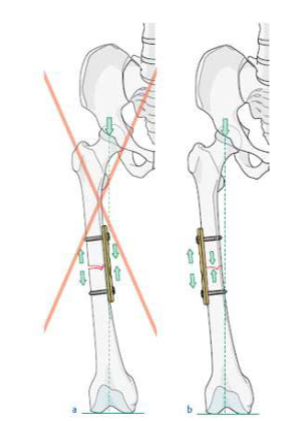
Fig. 6. a) A plate positioned on the side of compressive forces cannot neutralize the tension force, b) A tension band plate converts tensile force into compression on the opposite cortex [16].
Plates offer the benefits of anatomic reduction of the fracture with open techniques and stability for early function of muscle-tendon units and joints, but they must be protected from premature weight bearing. Disadvantages of plate fixation include the risk of bone re-fracture after their removal, stress protection and osteoporosis beneath a plate, plate irritation, and also in rare cases an immunologic reaction [10]. Plates neutralize deforming forces that cannot be counteracted by screws alone. Plates require countering to maintain optimum stability of the fracture reduction. The application of the screws is also critical because incorrect placement or sequence will result in displacement or shear and loss of reduction [10].
A researcher, by the name of Danis, was the first person to design and use a rigid longitudinal compression system that led to the healing of fractures without the formation of a visible callus [17]. This type of conventional rigid plating promotes primary healing through the direct compression of the plate to bone with or without a removable compressor and is now known as the AO/ASIF method (Association for the study of internal fixation). These techniques rely on the axial force generated by the insertion torque of spherical screw heads to compress the plate to the bone (see Fig. 7 a). It should be noted that not all plating devices rely on this method of compression. For instance, in the case of comminuted fractures, lag screws provide the necessary compression through the purchase of the distal bone fragment [17].

a b
Fig. 7. Conventional compression plates and locking dynamic compression plates, (a) Conventional plating relies on the axial force from the applied screw torque and the friction between the plate and bone to resist loading. (b) Locked plates do not require compression between the plate and bone due to the locking nature of threads [17].
Although inherently stable, DCPs also exhibit some major disadvantages. These include: the bending required prior to surgical insertion; the damage caused to vascular tissue adjacent to the bone; and most importantly stress shielding in the underlying bone [18]. Some researchers suggest that necrosis associated with vascular insufficiency, because of the application of bone plates, is the main cause of increased bone porosity [19]. Therefore, to decrease soft tissue damage, researchers sought the use of plating devices which limit the contact area between the plate and bone. A variation of the DCP, known as the LC-DCP or locked-compression DCP systems provides stability through locked tapered screw heads while limiting the plate-bone contact area (see Fig. 7 b). Another method commonly used to reduce stress shielding is to limit the difference between bone and plate rigidities through the use of more flexible plating materials. Early implants mainly consisted of stainless steel and vitallium alloys [20]. Interesting to note that there is still much controversy between the use of completely rigid systems and systems with increased material flexibility [21]. Some researchers believe that a small amount of micro-motion at the fracture site promotes more rapid fracture healing, because a combination of primary and secondary healing can occur in this case [22]. As it is a well-accepted concept, flexural rigidity is dependent both on the cross-sectional area and material properties of the plating device [23]. In terms of materials, less rigid titanium alloy devices with increased resistance to corrosion have proven to be advantageous [17], and are slowly beginning to replace stainless steel devices that previously dominated the market. Experimental evidence reveals an increasing trend towards the general acceptance of more flexible systems, as the level of stress shielding is reduced [24-26].
Specific plate designs include tubular plates, T- and L-plates, spoon plates, reconstruction plates, dynamic compression plates (DCP), locking compression plates (LCP), limited contact DCP (LC-DCP), and limited contact LCP (LC-LCP) (see Fig. 8). The many different types and designs of plates can be grouped functionally into four categories: neutralization plates, compression plates, buttress plates, and bridge plates [10].

Fig. 8. (left to right) DCP, LC-DCP, LC-LCP, Tubular plate, T-Plate, L-Plate and Reconstruction plate.
Neutralization plates are not a specific type of plates, but neutralization refers to how a plate functions in fracture fixation. A neutralization plate decreases the loading forces on a fracture by spanning the fracture and transferring the loading forces through the plate rather than through the fracture site. Neutralization plates are used in conjunction with inter-fragmentary screw fixation and neutralize torsional, bending, and shear forces. These are commonly used in fracture with butterfly or wedge-type fragment after interfragmentary screw fixation of the wedge portion of the fracture (Fig. 9). Stability of the plate is significantly improved by the inter fragmentary screw. Common fractures fixed with neutralization plates are wedge fractures of the humerus, radius, ulna and fibula [10].

Fig. 9. Wedge fractures with spiral or bending wedges can be reduced and fixed with plate and screws [12].
Compression plates, as the name indicates, are designed to apply compression to fractures. The standard compression plate is usually referred to as a dynamic compression plate (DCP), which is a misnomer since these plates supply static compression to a fracture. One method of producing compression is illustrated by the Fig. 10 (see next page please). The holes in the plate have sloped edges on the side of the hole distal from the fracture. A screw can be inserted in the hole at the end close to the fracture. This will lock the plate to the bone without moving the bone with respect to the plate. This is how the screw on the right of the plate in Fig. 10 was inserted. The screws on the left side of the plate are inserted at the far end of the hole with the shank of the screw touching the far end of the hole as shown in the top figure of Fig. 10. As the screw is inserted, the head will be forced to the right by the slope in the hole, moving the bone and its attached screw toward the fracture compressing it as shown in the bottom figure.
Buttress plates are employed to rigidly hold in place fractures at the end of long bones, especially at the knee and ankle, where the fracture site experiences large compressive and other kind of forces. In order to provide adequate fixation, these plates are broadened and carefully contoured at the joint end of the plate. For this reason, the buttress plates are referred to as peri-articular plates. The periarticular surfaces of long bone are complex having several surfaces at each joint. Buttress plates are contoured to a particular surface (medial, lateral, anterior, etc.), and so several different plate designs may be needed for an individual peri-articular region. There are a large number of buttress plates, and some of the more common configurations are T-shaped, L-shaped and bulbous end shaped plates. The contoured, periarticular portion of the plate offer these plates a three dimensional configuration.
Compression plates negate torsional, bending, shear forces and create compression across the fracture site either through especially designed self-compression holes in the dynamic compression plate (DCP) design. These holes exert compression through translation of the plate as the screw engages it [10].

Fig. 10. Spherical gliding principle: sphere (screw head) glides down inclined cylinder (screw hole); combination of downward and horizontal movement of the screw causes horizontal movement of underlying bone in respect to the stationary. This guarantees that compression will be exerted at fracture [10].
Buttress plates (see Fig. 11) negate compression and shear forces and frequently occur with metaphyseal-epiphyseal fracture, such as tibial plateau and tibial pilon fractures. They are frequently used in conjunction with inter-fragmentary screw fixation. They differ from other functional types of plates in that the plate is anchored to the main stable fragment, but not necessarily to the fragment it is supporting [10].

Fig. 11. Different buttress plates for plateau fractures. A) L-plate on lateral plateau, B) T-plate on medial plateau, C) Lateral tibial head plate[10].
Bridge plates (see Fig. 12) are used to span a comminuted unstable fracture or bony defect in which an anatomic reduction and rigid stability of the fracture cannot be restored by fracture reduction. This function is the hardest for a plate to maintain. Biologic additions to this form of fixation frequently are required in the form of autogenous bone grafting [10]. After adequate bone regeneration has occurred, implant removal may be indicated because of patient preference, or to restore skeletal strength. The risk of re-fracture after plate removal can be minimized by evaluating multiple roentgenographic views of the fracture. Restoration of the medullary canal and obliteration of all fracture lines suggest adequate healing, although re-fracture through screw holes may still occur [10].

Fig. 12. A) Bridge plate used to bridge zone of comminution maintains length and rotational alignment by “splinting”, rather than compression. Union depends on formation of callus. B) Wave plate (Weber) allows addition of bone graft under plate [10]
Interlocking Nails (Rods):
Over the past 50 years medullary nail techniques for fracture fixation have gained universal acceptance. The conventional Kuntscher nail is a longitudinally slotted tube that is inserted into a long bone under pre-stress. The radial stress caused by the insertion of an intramedullary nail leads to friction between nail and bone that secures the relative movement between the two nailed fragments. However, since these frictional forces are limited, the application of this technique is limited just to simple midshaft diaphyseal fractures[27]. The intramedullary rod is a commonly used device in long-bone fractures, particularly in the lower limb fracture. It offers the advantage that it can be implanted through a small incision and without considerable surgical exposure. Closed interlocking nail fixation is the procedure of choice for femoral shaft fractures, especially in poly-trauma patients. This treatment modality has been the subject of controversy since its introduction because of concerns of damage to the medullary circulation, possibilities of embolism, and complications from misapplications of the technique because of a lack of understanding of the biomechanical principles of medullary nail fixation [10].
Various types of medullary nails are centromedullary nails, condylocephalic nails, cephalomedullary nails, centromedullary interlocking nails, and cephalomedullary interlocking nails. Centromedullary nails enter the bone in line with the medullary canal. They obtain contact with the bone through multiple points of longitudinal interference. They depend on restoration of bony contact and stability to avoid axial and rotational deformation of the fracture. Condylocephalic nails enter the bone in the condyles of the metaphyseal-epiphyseal area. They frequently are inserted in groups for added rotational stability. Cephalomedullary nails have a centromedullary portion, but also permit fixation up into the femoral head [10].
A perfect medullary nail has not yet been designed. The varying contour of bones make invention of such a nail actually impossible, but improvement in the design of medullary nails should seriously continue. A medullary nail should meet the following requirements[10]:
It should be strong enough and provide sufficient stability to maintain alignment and position, including prevention of rotation; it should include interlocking transfixing screws as necessary;
It should be constructed so that contact-compression forces may impact the fracture surfaces, a desirable physiologic stimulus to union; and
It should be placed so that it is accessible for easy removal; attachments are provided to facilitate removal.
An Interlocking Nail (IN) is basically an intramedullary pin secured in position by proximal and distal transfixing screws which engage the bone to the nail to provide axial bending and torsional stability (see Fig. 14). This procedure, in man, generally involves power reaming and insertion by closed technique on a distraction table under fluoroscopic control[10]. Interlocking fixation is defined as dynamic, static, and double locked. Dynamic fixation controls bending and rotational deformation, but allows nearly full axial load transfer by bone. Dynamic fixation is used in axially stable fractures and some nonunions. Static fixation controls rotation, bending, and axial load and makes the implant a more load-bearing device with the potential for a reduced fatigue life. It is especially useful in comminuted, nonisthmal fractures of the femur and tibia. The double-locked mode controls bending, rotational forces, and some axial deformation, but because of capability of axial translation of the screw within the nail, some shortening is possible. This mode of fixation is used in fracture of the humerus and occasionally in delayed unions and nonunions [10].

Fig. 7. Interlocking nails inserted into the medullary canal, a)Femoral nail b)Tibial nail.[28, 29]
The stability of fracture fixation by nailing greatly depends on the mechanical properties of the nail, the nail's fit in the medullary space, and the mechanical properties of the locking screws or bolts. The torsional and bending stiffness of the nailed bone mainly depends on the diameter of the nail. Nails with a larger diameter offers a significantly greater stiffness compared to that of a small diameter [27].
Implant Materials:
The most commonly used metals and polymers for orthopaedic applications are stainless steel, cobalt-chromium alloys, titanium alloys, ultrahigh molecular weight polyethylene (UHMWPE), and polymethyl methacrylate (PMMA). Interesting to know that stainless steel and cobalt-chromium alloys have similar elastic moduli (~200 GPa), which are about twice the modulus of titanium alloys (~ 100 GPa), and an order of magnitude stiffer than the typical cortical bone (say 20 GPa), which is in turn an order of magnitude stiffer than either UHMWPE or PMMA. In addition, cobalt-chromium and titanium alloys are typically less ductile and stronger than stainless steels. The properties of medical grade stainless steel provide a balance between high strength, ductility, and fatigue performance. Ductility is essential for fracture fixation components, since they should be deformed in the operating room to conform to the bone curvature at the fracture site. Corrosion resistance is another important consideration, which is achieved in stainless steels by keeping the carbide content to a minimum and by including chromium. Resistance to pitting and crevice corrosion is also very important and can increase with increasing chromium, molybdenum, and nitrogen. When components are manufactured by stainless steel, cobalt, chromium nickel oxide layer is formed by passivation of the devices in a strong nitric acid bath. If the protective layer is scratched, no serious problem arises since the film can reform. This oxide layer increases the in vivo corrosion resistance by offering a passive layer between the tissues and bulk material. The titanium based material most commonly used in orthopaedic applications is titanium-aluminum-vanadium alloy, Ti-6Al-4V. Unalloyed pure titanium can be used for implants where high stresses are not expected, e.g. fracture fixation devices for wrists and hands. But, if high strength is needed, titanium alloys must be employed. An adherent layer of titanium oxide (TiO2) provides corrosion resistance that greatly exceeds that of stainless steel and the cobalt alloys, even in saline solutions. It also has excellent resistance to pitting, intergranulkar and crevice corrosion. Moreover, the oxide layer of titanium and its alloys offer excellent interfaces with bone, which can grow right up to the implant surface without considerable fibrous tissue formation that can be seen in other metal-bone interfaces. Another advantage of titanium implants is their biocompatibility from the stress shielding point of view (because of their lower modulus of elasticity compared to that of stainless steel and cobalt-chromium alloys). It should be noted that Ti6Al4V alloy is a notch-sensitive materials, and without additional surface treatment, it shows very poor wear resistance compared to stainless steel and cobalt-chromium alloys [30].
Sample Products of Pooyandegan Pezeshki Pardis (3P):
The following figures are pictures from some of 3P Factory products including screws (Fig. 15), plates (Fig. 16), and nails (Fig. 17).
Fig. 15. Bone screws a. Cancellous screw, b. Cortical screw, c. Locking screw

Fig. 16. Bone plates, a. Locking compression plate (LCP), b. Dynamic hip screw (DHS)

Fig. 17. Intramedullary Nails, a. Femoral nail, b. Tibial nail.
Acknowledgement: The authors would like to express their heartfelt appreciation to Mr. Farshid Emari Alah Yari, the respected Managing Director of 3P Factory, and also to Mr. Ali Emari Alah Yari, the respected Managing Director of the Consortium of Iranian Medical Implants Pioneers Company for their continuous and kind support.
📌 FAQ (x5)
Q1. What are orthopedic implants and why are they used? | အရိုးတု အင်ပလန့်များ ဆိုသည်မှာ အဘယ်နည်း၊ မည်သည့်အတွက် အသုံးပြုကြသနည်း။
Orthopedic implants are medical devices used to replace or fix bones or joints. They are mainly made from stainless steel and titanium alloys, designed to restore stability and assist in bone healing. | အရိုးတု အင်ပလန့်များသည် အရိုးများ (သို့မဟုတ်) အဆစ်များကို အစားထိုးရန် သို့မဟုတ် ပြင်ဆင်ရန် အသုံးပြုသော ဆေးဘက်ဆိုင်ရာ ပစ္စည်းကိရိယာများ ဖြစ်သည်။ ၎င်းတို့ကို များသောအားဖြင့် သံမဏိနှင့် တိုက်တန်နီယမ်စပ် သတ္တုများဖြင့် ပြုလုပ်ထားပြီး အရိုး၏ တည်ငြိမ်မှုကို ပြန်လည်ရရှိစေရန်နှင့် အရိုးဆက်စပ်မှုကို ကူညီရန် ဒီဇိုင်းထုတ်ထားသည်။
Q2. What are the three main types of orthopedic implants? | အဓိက အရိုးတု အင်ပလန့် အမျိုးအစား (၃) မျိုးကား အဘယ်နည်း။
The main types are orthopedic screws, orthopedic plates, and orthopedic nails (rods). | အဓိက အမျိုးအစားများမှာ အရိုးသုံး ချုပ်ရိုးဝက်အူများ၊ အရိုးပြားများ (Plates) နှင့် အရိုးတွင်းချောင်းတိုင်များ (Nails/Rods) ဖြစ်သည်။
Q3. What are the stages of bone healing after a fracture? | အရိုးကျိုးပြီးနောက် ကုသမှု အဆင့်ဆင့်မှာ ဘာတွေလဲ။
The bone healing process has three stages: 1) Inflammation, 2) Bone production (soft callus → hard callus), and 3) Bone remodeling. | အရိုးကျိုးပြီး ပြန်လည်ကောင်းမွန်လာမှုတွင် အဆင့်သုံးဆင့် ရှိပါသည်- (၁) ရောင်ရမ်းခြင်း၊ (၂) အရိုးအသစ်ဖြစ်ပေါ်ခြင်း (ပျော့သည့် ကယ်လ်ဆပ် → မာသည့် ကယ်လ်ဆပ်)၊ နှင့် (၃) အရိုးပြန်လည်ပုံဖော်ခြင်း။
Q4. What is stress shielding in orthopedic screws? | အရိုးသုံးဝက်အူများတွင် ဖြစ်တတ်သော 'စတရက် ရှီလ်ဒင်' ဆိုတာ ဘာလဲ။
Stress shielding occurs when metallic screws carry most of the load, reducing stress on surrounding bone, which may cause bone resorption and weakening. | 'စတရက် ရှီလ်ဒင်' ဆိုသည်မှာ သတ္တုဝက်အူများက ဝန်အားအများစုကို သယ်ဆောင်မိသွားပြီး ပတ်ဝန်းကျင်ရှိ အရိုးပေါ်သို့ သက်ရောက်မည့် အားကို လျှော့ချမိသည့် ဖြစ်စဉ်တစ်ခုဖြစ်ကာ ၎င်းကြောင့် အရိုးပါးခြင်းနှင့် အားနည်းခြင်းများ ဖြစ်ပေါ်စေနိုင်သည်။
Q5. What materials are commonly used in orthopedic implants? | အရိုးတု အင်ပလန့်များ ပြုလုပ်ရာတွင် အသုံးများသော ပစ္စည်းများမှာ ဘာတွေလဲ။
Common materials are stainless steel, cobalt-chromium alloys, titanium alloys, UHMWPE, and PMMA. | အသုံးများသော ပစ္စည်းများမှာ သံမဏိ၊ ကိုဘော့-ခရိုမီယမ်စပ် သတ္တု၊ တိုက်တန်နီယမ်စပ် သတ္တု၊ UHMWPE (အလွန်မြင့်မားသော မော်လီကျူးအလေးချိန်ရှိ ပိုလီအီသီလင်း) နှင့် PMMA (အရိုးကပ်စေသော ဓာတုပစ္စည်း) တို့ ဖြစ်သည်။
🦴 Compression Side vs. Tension Side
When a bone is subjected to bending forces, it experiences two opposite types of mechanical stress:
a) Compression Side | ဖိအားသက်ရောက်ရာ ဘက်ခြမ်း
This is the side of the bone where forces push inward, compressing the tissue.
The bone becomes shorter and thicker on this side.
Example: When you bend a stick, the inner curve is under compression.
b) Tension Side ✅ | ဆွဲဆန့်အားသက်ရောက်ရာ ဘက်ခြမ်း ✅
This is the side where forces pull outward, stretching the bone.
The bone becomes longer and thinner on this side.
Example: On the same bent stick, the outer curve is under tension.
✅ Tension side is more prone to fractures, especially in bones like the femur or tibia, because bone is generally weaker under tension than compression.
🧠 Why This Matters in Orthopedics
Implant placement often considers which side is under tension vs. compression.
Plates and screws are commonly placed on the tension side to counteract the pulling forces and prevent failure.
Understanding these forces helps surgeons choose the right fixation method and location.

✅ Confirmed! I've found images that clearly show a neutralization plate in orthopedic use—this type of plate is specifically designed to reduce torsional (twisting), bending, and shear forces across a fracture site. It works by neutralizing the mechanical stresses that would otherwise act on the bone, especially when combined with lag screws.
You can view detailed examples and surgical applications in these resources:
- Neutralization plate with lag screw for humerus fracture
- Neutralization plate for metatarsal wedge fracture
- Neutralization plate for lateral ankle fracture

A lag screw is a specialized orthopedic screw used to compress two bone fragments together, promoting stable fixation and direct bone healing.
🔧 How a Lag Screw Works ( lag=draw together )
- It is threaded only at the tip (distal end), while the shaft near the head is smooth.
- The near cortex (closest bone layer) is drilled wider so the screw slides freely through it.
- The far cortex (deeper bone layer) is drilled to match the thread diameter so the screw grips it.
- When tightened, the screw pulls the two bone fragments together, creating interfragmentary compression.
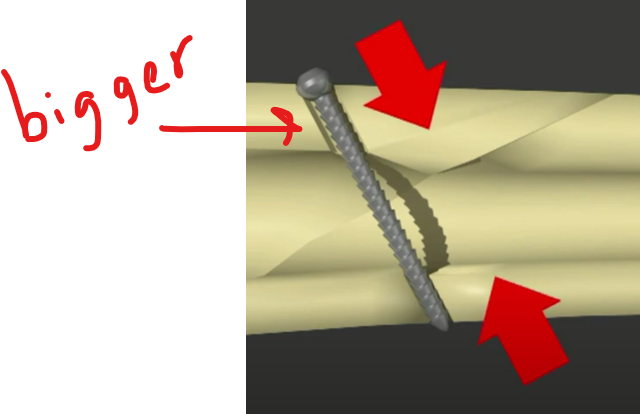

🦴 Key Features
| Feature | Function |
|---|---|
| Partially threaded | Allows sliding through near cortex |
| Glide hole | Prevents thread engagement in near cortex |
| Thread hole | Anchors screw in far cortex |
| Compression effect | Promotes absolute stability and bone healing |
📌 Common Uses
- Oblique fractures of long bones (e.g., tibia, humerus)
- Articular fractures needing anatomical reduction
- Often used with plates (e.g., neutralization plate) to enhance stability
You can explore detailed surgical techniques and visuals on AO Foundation’s Lag Screw Principles. Let me know if you'd like a diagram showing how the glide hole and thread hole work together!
Dynamic Compression Plates (DCP) and how they function in orthopedic surgery. These visuals show:
- The sloped screw holes that allow for controlled compression when screws are inserted eccentrically
- The difference between standard DCP and Limited Contact DCP (LC-DCP), which reduces bone-plate contact to preserve blood supply
- How DCPs are used to stabilize fractures and provide static compression, despite the name
https://www.youtube.com/watch?v=aguI3kdXS7o

“interlocking nail” comes from the locking mechanism that secures the nail inside the bone—it's not just a rod, it's a rod that locks into place using screws.
🔩 Why It's Called “Interlocking”
- The nail is inserted into the medullary canal (the hollow center of a long bone).
- At both ends of the nail—proximal and distal—there are holes designed for locking screws.
- These screws pass through the bone and into the nail, effectively “interlocking” the nail with the bone.
This locking system:
- Prevents rotation and shortening of the fractured bone
- Provides axial and torsional stability
- Allows early weight-bearing and faster healing
🦴 What Makes It Special
| Feature | Function |
|---|---|
| Locking screws | Secure the nail to the bone |
| Load-sharing design | Bone and nail share mechanical stress |
| Minimally invasive | Inserted through small incisions |
| Used in long bones | Femur, tibia, humerus |
So, the name “interlocking nail” reflects its mechanical locking function, which is the key to its stability and success in treating long-bone fractures.
Would you like a visual diagram showing how the screws lock into the nail and stabilize the fracture? I can help sketch that out for you.
UHMWPE stands for Ultra-High Molecular Weight Polyethylene—a super tough, wear-resistant plastic widely used in orthopedic implants, especially in joint replacements like hips and knees.
There are no comments for now.